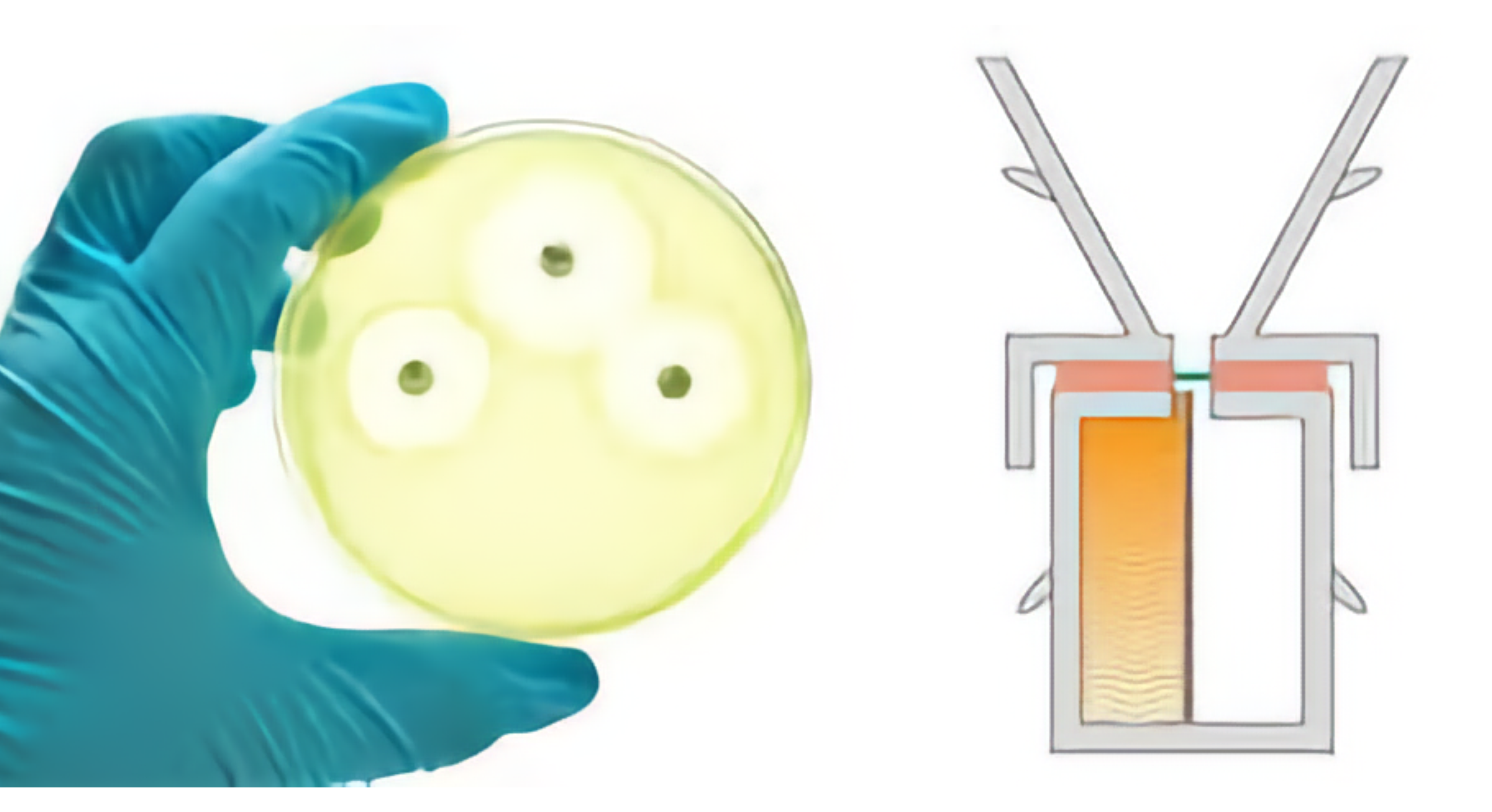
Evaluating Irritation, Metabolism, and Barrier Integrity
At MedPharm, we utilize Reconstructed Human Epithelium (RHE) models to assess the irritation potential, metabolic activity, and barrier integrity of topical and transdermal formulations. These advanced in vitro models provide highly predictive, reproducible, and regulatory-compliant data, assisting developers in optimizing formulations, minimizing risks in clinical studies, and meeting regulatory safety requirements—all while reducing the reliance on animal testing.
Our scientifically validated RHE models mimic the structural and functional properties of human skin and mucosal tissues, making them essential tools for the evaluation of pharmaceutical, cosmetic, and dermatological products.
Why RHE Testing Matters in Drug Development
Ethically Responsible and Regulatory Accepted: Provides animal-free, human-relevant data to support FDA, EMA, and ICH guidelines.
Highly Predictive for In Vivo Performance: Mimics the physiology, metabolism, and barrier function of actual human tissues.
Essential for Safety and Irritation Studies: Evaluates skin and mucosal irritation, corrosion, and toxicity.
Supports Drug Metabolism and Transport Studies: Assesses enzyme activity, drug metabolism, and permeability.
Ideal for Topical, Transdermal, and Mucosal Products: Supports dermatological, ophthalmic, respiratory, and vaginal drug delivery systems.
How RHE Testing Works
MedPharm employs live human epidermis and mucosal epithelium models to evaluate drug absorption, metabolism, irritation, and inflammatory responses:
- Test Compound Application: The formulation is applied to RHE tissues, simulating clinical application.
- Irritation and Toxicity Assessment: Tissue viability, cytokine release, and histological analysis determine irritation potential.
- Metabolism and Enzyme Activity Analysis: Drug metabolism is assessed through enzyme expression and metabolite identification.
- Barrier Function and Permeability Testing: Transepithelial electrical resistance (TEER) and dye penetration assays evaluate barrier integrity.
MedPharm’s RHE Testing Capabilities
Skin Irritation and Corrosion Testing: Predicts irritancy potential for drug, cosmetic, and chemical formulations.
Mucosal Irritation and Permeation Testing: Evaluates buccal, vaginal, nasal, and ocular formulations.
Drug Metabolism and Enzyme Activity Studies: Assesses metabolic stability and bioactivation in human-like tissue models.
Barrier Integrity and Permeability Analysis: Measures transepithelial resistance (TEER) and membrane disruption.
Inflammatory Response and Cytokine Profiling: Determines pro-inflammatory cytokine release (IL-1α, IL-8, TNF-α).
Key Applications of RHE Testing
Safety and Irritation Studies: Determines irritancy potential for regulatory approval and product optimization.
Transdermal and Topical Drug Metabolism: Evaluates enzyme activity and drug breakdown in the skin.
Bioavailability and Permeation Studies: Assesses drug penetration and systemic absorption.
Mucosal and Ophthalmic Drug Delivery: Supports nasal, vaginal, buccal, and ocular formulation development.
Cosmetic and Dermatological Testing: Verifies biocompatibility and efficacy of skincare products.
Why Choose MedPharm for RHE Testing?
- Industry-Leading Expertise in In Vitro Skin and Mucosal Models
- Validated, Regulatory-Compliant RHE Assays (OECD, FDA, EMA, ICH)
- State-of-the-Art 3D Tissue Engineering Capabilities
- Comprehensive Data Packages for IND, NDA, ANDA, and Global Filings
- Customizable Study Designs for Unique Formulations