MedPharm offers customers options for testing in vitro permeation and penetration testing (IVPT) using its proprietary, fully automated flow-through system (MedFlux-HT®) or classic static vertical diffusion cells (i.e. Franz cells). IVPT is increasingly accepted by regulatory authorities as part of the justification for generic biowaivers, used to justify patentable differences between formulations, and as a screen during formulation optimization and selection for new chemical entities.
IVPT is used to measure the permeation and penetration of a drug into and through tissue. It is an essential tool for optimizing topical and transdermal formulations, and cost-effectively provides a measure of performance of a product using human tissue.
MedPharm has spent years perfecting procedures to minimize variation in this complex technique. When IVPT is used to make critical preclinical decisions, justify formulation selection, or demonstrate bioequivalence in a topical generic product, regulatory authorities insist on a rigorous level of validation that can only be achieved after considerable experience.
IVPT is a cost-effective method used to demonstrate:
- The optimization and characterisation of formulation performance
- De-risking formulations prior to expensive time-consuming clinical trials
- Product performance and new IP to potential investors
- The bioequivalence of a generic compared to an originator product
- New marketing claims to educate key opinion leaders
Download our study – Method improvement in IVPT: Reducing the variability of an inherently variable technique
MedPharm has different approaches for IVPT:
Ex vivo, small, or diseased tissue
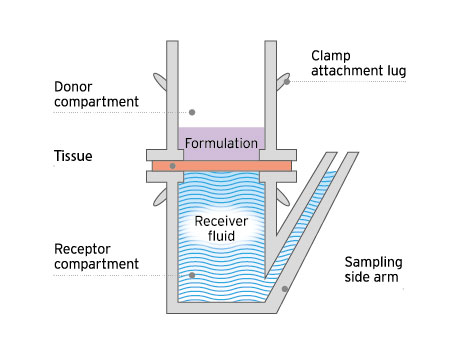
The classic static cell used for ex vivo permeation and penetration testing.
These studies utilize static or Franz diffusion cells, and can be adapted to test small or diseased tissues.
These studies can be used with tissue that may be more difficult to source such as:
- Healthy human skin (surgical and cadaver)
- Mucosal Tissue (e.g. Buccal, esophageal, vaginal, anal, rectal)
- Eyelids
- Cornea and sclera of the eye
- Hyperkeratized skin
- Psoriatic plaque biopsies
- Non-melanoma skin cancers (e.g. BCC, AKs, and SCC)
High throughput Skin Permeation
MedPharm has developed an automated high throughput flow-through diffusion cell system (MedFlux-HT®) to more closely mimic the clinical situation and speed up IVPT experiments. MedFlux-HT® employs a continuous flow of receiver fluid under the tissue to mimic blood flow while automating sample collection directly into 96-well plates. This system is coupled with liquid robotics for sample preparation that can be injected directly onto LC-MS/MS. The optimized fluidics of this system ensure sink conditions for highly lipophilic compounds, reduces variation associated with traditional manual sampling, while increasing the throughput of an in vitro permeation testing.
The significant benefits of MedFlux-HT®:
- increased confidence in clinical performance, in particular, for lipophilic drugs
- high throughput sample collection for more accurate formulation kinetics
- advantages in regulated studies that require precise permeation profiles
- reduction in testing variation
- increased opportunity to note patentable differences between formulations
- more credible evidence to support marketing claims
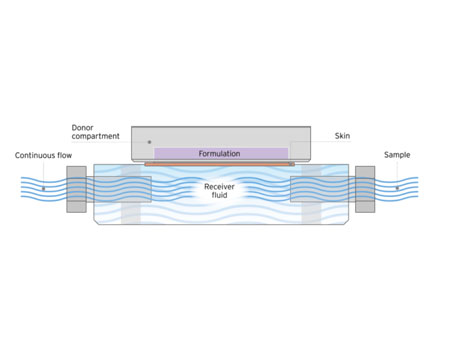
A unique flow-through method for ex vivo permeation and penetration testing. MedFlux-HT incorporates an automated higher throughput platform.
Hard Tissue/Nails
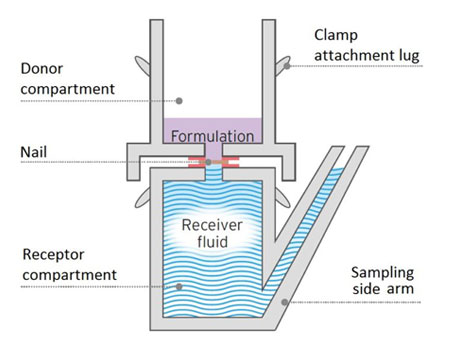
MedPharm’s unique ChubTur® model is a static cell specific for measuring permeation and penetration through hard tissue such as nails.
Measuring the permeation and penetration of a drug through toe or fingernails and other hard tissues present specific challenges. MedPharm’s unique ChubTur® model is a modified static cell specifically designed to measure the permeation and penetration of a drug through hard tissues such as nails. This proprietary validated model is widely accepted by regulatory authorities as the gold standard for modelling penetration through nails.
ChubTur® studies involving nails led to the first approval for demonstration of bioequivalence without the requirement of any clinical testing.
MedPharm has also developed a device called the TurSh™ which is used in combination with ChubTur® cells to explore the depth of permeation of drug substance within the nail.
Reconstructed Human Epithelium or living tissue (eye, nasal, lung, gut, mouth, mucosal membranes)
When measuring the permeation and penetration of a drug through epithelium without a protective barrier like skin, MedPharm employes reconstructured human epithelium. The tissue mimics the living epithelium to capture active and passive transport, cilia, mucous secretion, and/or the same permeability profile of intact human tissue. The tissue has the added advantage of moving out of animal tissue that can be damaged or ‘leaky’ as a part of the sample collection and processing procedures.
The significant benefit of using reconstructed human epithelium is:
- human derived to refine, reduce, and replace animal use
- better predictor of the in vivo clinical situation
- allows for active and passive transport in relevant tissue

Download our poster, Reconstituted Nasal Epithelium as a Model for Nasal Drug Delivery, to learn more about the uses and benefits of our reconstructed nasal tissue.

