Specialized Expertise
As a world-class organization with a proven track record, we have over 20 years of experience in formulation development. We believe in applying the specialized expertise of our team to deliver the most technically challenging formulations in topical and transdermal development.
We provide the necessary knowledge, services and tools to understand the different conditions that can affect each project in order to develop an exceptional formulation that is stable, safe and compliant.
MedPharm’s close partnership and open dialogue with regulatory bodies provides a deep understanding of the regulatory landscape, helping to accelerate the approval process for your therapeutics.
With over 55 market approved products globally, we are a proven scientific expert and advisor trusted by the industry’s regulatory bodies, investors and key opinion leaders.

Rigorous Approach
Our reputable and high quality proprietary methods successfully support our customers’ business and scientific needs. We stand out from the crowd by having a philosophy which links formulation development closely with drug and product design. MedPharm’s approach is fundamentally based on the QbD principles laid out in ICH Q8. This approach is applied to all areas of our development process, helping to mitigate risks and significantly reduce costs as you progress through the drug product development pipeline.

We work with our clients to successfully establish their target product profiles. Our integrated approach to topical and transdermal drug delivery gives us a clear line of sight on the project, with key final product characteristics incorporated from an early stage in development. Understanding elements such as route of delivery, site of action and even target shelf life of a drug product ensures that our clients’ development projects are cost and time-effective.
Problem Solvers
Our extensive experience in topical and transdermal formulation development enables us to anticipate the challenges our customers can face along the way. Importantly, MedPharm offers the flexibility to design custom solutions that answer our clients’ evolving requirements. We propose the optimal program for their current circumstances to maximize the chances of achieving their desired outcome.
MedPharm’s experienced team adapts to your internal and external processes to deliver a high standard of science, with end-to-end services covering you from formulation development to clinical manufacturing. We believe in going above and beyond to develop innovative solutions based on client needs. By working together and applying our unparalleled resources to each project, we can add long term value and meet your evolving requirements. Overall, our approach to formulation development helps to efficiently mitigate many of the risks associated with any project.
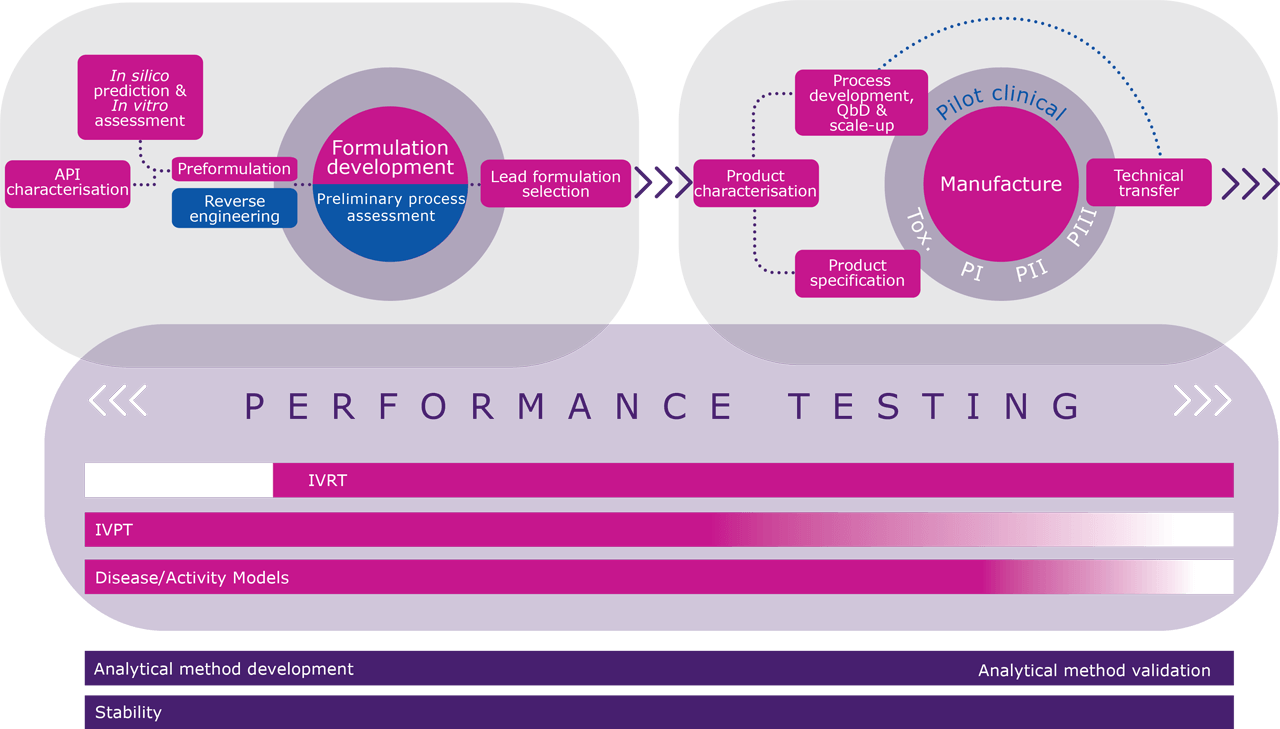
Click on the interactive elements below to see how MedPharm can support you at each stage of the development process.